Autophagy - An Overview
Within the context of this Cleanse, the importance of supporting the process of autophagy cannot be overstated. Kai Sun, et al. define this process as ‘a dynamic degradation and recycling system that provides biological materials and energy in response to stress.’(Sun, Kai et al. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell & bioscience.(2013). ) As mentioned earlier, this process is ongoing and it provides key support in the prevention of the accumulation of potentially toxic cellular debris. The success of this critical self cleansing apparatus is dependent on the level of functionality at each step.
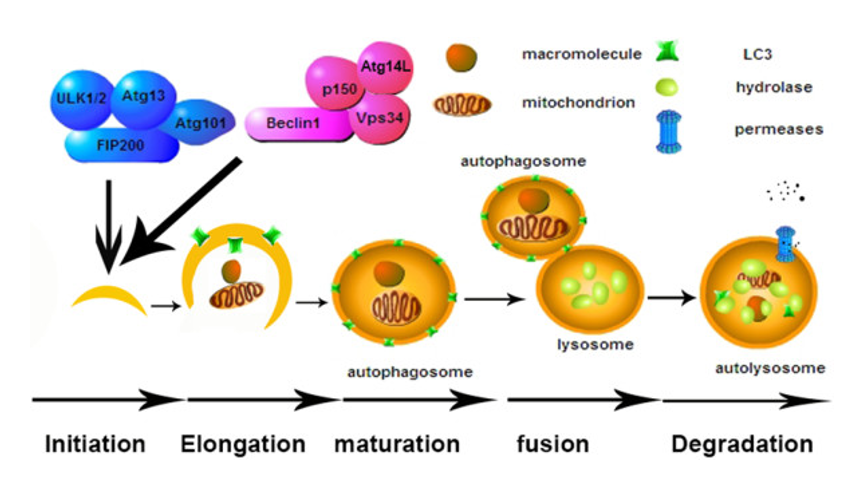
Autophagy includes five steps: initiation/nucleation, elongation, maturation, fusion and degradation. Firstly, the material in need of removal, which mainly include macromolecules and organelles, are encompassed by a double-membrane vesicle that gradually extends and ultimately forms autophagosome. Then autophagosome fuses with lysosome to form autolysosome, where the materials are degraded by lysosomal hydrolase and the productions are recycled back to cytoplasm by lysosomal permease. Ultimately, the goal of autophagy is to promote cell survival by elimination of damaged organelles and protein aggregates, as well as by facilitating bioenergetic homeostasis. With that in mind, it stands to reason that alterations or deviations from normal can lead to an acceleration of the aging process. Stimulating autophagy is accomplished through a variety of means; physical activity, caloric restriction and intermittent fasting. When looking at the cellular processes involved, two regulatory proteins play a key role, mTOR and AMP-activated protein kinase (AMPK). In short, when nutrient levels are high and there is abundant nourishment, mTOR is activated, which in turn shuts down autophagy; the opposite happens when mTOR is inhibited, autophagy is activated. Therefore, much focus has been placed on inhibiting mTOR. However, we must be cautious as the need for balance is key to mTOR relative to metabolic function- appropriate mTOR activation protects pancreatic β-cells against cholesterol-induced apoptosis, promotes the differentiation of adipocytes, preserves endothelial cell function during hyperglycemia, and maintains glucose homeostasis. Similarly, increasing AMPK, through things like high intensity exercise as well as nutrient restriction and targeted nutritional therapy, can stimulate autophagy, in addition to providing other physiologic benefits such as insulin sensitizing effects.
Autophagy Perspective - A Hallmark of Aging
Autophagy has been found to naturally decrease as we age, and because of this diminished activity the accumulation of damaged macromolecules and organelles during aging is observed. Interestingly, when failure exceeds a certain threshold, it has been reported that aging-associated diseases, such as neurodegeneration or cancer, among others, can be exacerbated and worsened. Conversely, it has been seen in different organisms that the maintenance of a proper autophagic activity can be directly correlated to extending longevity.
Despite many theories of aging, perhaps the most popular theory is the Free Radicals (or Oxidative Stress) Theory of Aging, which hypothesizes that an accumulation of Reactive Oxygen Species (hereafter ROS) facilitates the oxidative damage of biomolecules, ultimately resulting in a decline in cellular function. (Kamel HK, Mooradian AD, Mir T. Biological theories of aging. Disease-a-Month (2015) 61:460–6.) A substantial body of evidence does, in fact, support this theory.
The intertwining of the ROS theory of aging and autophagy relative to elements such as proteostasis, mitochondrial function and genomic instability, among others. First, when it comes to proteostasis, this serves as one of the major functions of autophagy within normal tissues. Imbalance of proteostasis, however, due oftentimes to excessive oxidative stress and impaired autophagy leads to protein aggregation, accumulation of misfolded proteins and in the end to cellular dysfunction. In fact, research has shown that neurodegenerative diseases share many attributes, including the accumulation of ROS, misfolded proteins, and damaged organelles, which is a direct result of decreased autophagy activity.
Autophagy is directly tied closely to mitochondrial function. The subprocess within autophagy relative to mitochondria is referred to as mitophagy. This is necessary in normal differentiation of certain cell types such as red blood cells, in embryogenesis, immune response, cell programming, and cell death. And while mitophagy is required not only to remove damaged mitochondria, it is also vital in order to promote the biosynthesis of new mitochondria, which, in turn, ensures quality control over the mitochondria.
Finally, when it comes to genomic stability, several studies have demonstrated that autophagy represents a “safeguard” of genome stability both directly (DNA repair modulation) and indirectly.

Autophagy