Background
Many studies have shown that omega-3 fatty acids (OM3), specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have numerous health benefits, particularly in cardiovascular health. (1-9) However, the bioavailability and clinical efficacy of Omega-3 supplements depend on various factors, including the form of the Omega-3, the food matrix effects, and the metabolic capacity of the individual. The most common forms of Omega-3 supplements on the market are esterified in triglycerides (TG) or ethyl esters (EE); however, these forms have been shown to have lower absorption rates and may cause gastrointestinal side effects. (10-13)
A study measuring the Omega-3 Index levels of the Canadian population found that the majority of Canadian adults have an Omega-3 Index below the recommended level of 8%. Furthermore, studies suggest that obese individuals have even lower Omega-3 Index levels which highlights the importance of Omega-3 supplementation in the population, particularly for those who are obese or have lipid malabsorption issues. (14)
Recent studies have shown that the monoglyceride (MAG) form of Omega-3 (MAG-O3™) is a more effective carrier of Omega-3 compared to TG and EE forms, particularly for populations that suffer from lipid malabsorption or obesity.
Bioavailability in a Healthy Population
Clinical Bioavailability Study 1
The first study, a randomized double-blinded crossover trial, compared the pharmacokinetics of Omega-3 supplements in monoacylglycerol or ethyl ester in humans. The study found that after receiving the MAG form (MAG-O3), plasma EPA and DHA peaked at a concentration 3 and 2.5 times higher, respectively, than with the EE form. When provided in MAG form, omega-3 plasma concentration during the absorption phase was on average 3-5 times higher than in EE form. When Omega-3 were provided esterified in MAG, their concentration 24 hours post-dose was higher than in EE. The study concluded that plasma concentration of DHA and EPA was higher when provided in MAG (MAG-O3) than EE form. (15)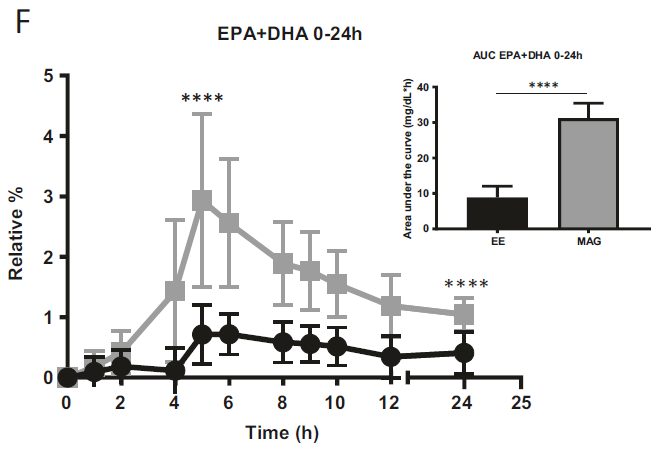
Figure 1: Relative % of plasma EPA + DHA in total lipids over 24 h. All results are expressed as mean ± SD (n = 20).
Clinical Bioavailability Study 2
The second study, a randomized double-blinded crossover trial, compared the 24-h AUC of the plasma concentrations of EPA, DHA, and EPA+DHA when provided esterified in monoglycerides (MAGs), EEs, or TGs, (primary outcomes) and evaluated their side effects over 24 h (secondary outcome). The study found that the 24 h AUC of plasma EPA was approximately 2 times and 1 time higher after the MAG compared with the EE and TG forms of Omega-3, respectively. The study concluded that the plasma OM3 FA concentration in adults is greater after supplementation with Omega-3 FAs esterified in MAGs rather than in EEs or TGs, suggesting that with a lower dose of MAG Omega-3 (MAG-O3) FAs, the plasma Omega-3 FA concentrations attained are similar to those after higher doses of Omega-3 FAs esterified in EEs or TGs. (16)
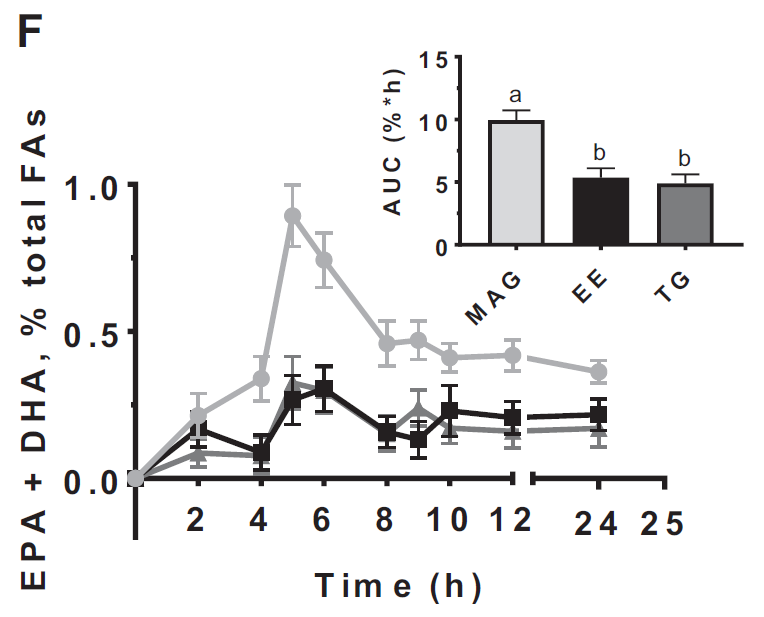
Figure 2: Relative plasma EPA+DHA concentrations in adults in the 24 h after
taking MAG, EE, and TG n–3 FA supplements.
Clinical Bioavailability Study 3
The third study, a randomized trial, evaluated the impact of three different fish oil forms (EE, rTG and MAG) on the Omega-3 Index after 8 weeks of daily supplementation. The study found that the Omega-3 Index at 8 weeks was 8.5 for the monoglycerides (MAG-O3™) group, 7.7 for the re-esterified triglyceride (rTG) group, and 7.1 for the ethyl ester (EE) group. The study concluded that Omega-3 Index is greater after daily supplementation for 8 weeks with Omega-3 FAs esterified in monoglycerides (MAG-O3™) rather than in EEs or rTGs. Only the Monoglycerides (MAG-O3) group was able to achieve an Omega-3 Index of 8.0 with a daily dose of 1.3 g of EPA+DHA. (17)
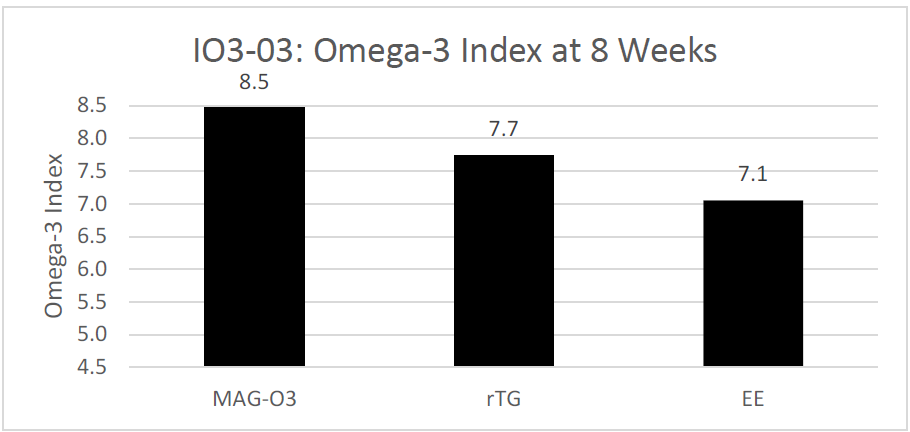
Figure 3: Red Blood Cells (Omega-3 Index) levels after 8 weeks of supplementation of MAG-O3™, EE and TAG forms of EPA and DHA. Results are expressed in %.
Clinical Studies in a Lipid Malabsorption or Obesity Population
Clinical Bioavailability Study 4
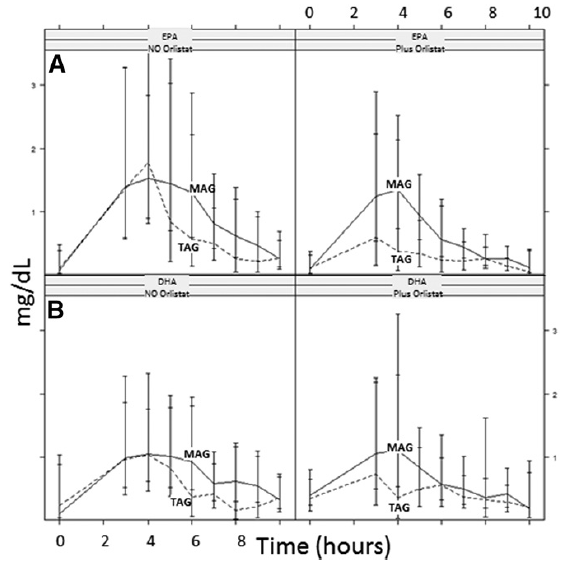
The fourth study, a double-blinded clinical trial, examined the accretion of EPA (500 mg/day) and DHA (300 mg/day) when consumed as TAG or MAG (MAG-O3™), into the erythrocytes, plasma, and chylomicrons of 45 obese (BMI ≥30 kg/m2 and ≤40 kg/m2) volunteers who were and were not administered Orlistat, an inhibitor of pancreatic lipases. The study found that intake of MAG-enriched oil (MAG-O3™) resulted in higher accretion of Omega-3 than with TAG, the concentrations of EPA and DHA in erythrocytes being, respectively, 72% and 24% higher at 21 days (P < 0.001). In addition, MAG-O3 increased the plasma concentration of EPA by 56% (P < 0.001) as compared with TAG. In chylomicrons, MAG intake yielded higher levels of EPA with the area under the curve (0-10 h) of EPA being 55% greater (P = 0.012). These results indicate that in obese human subjects with Orlistat-induced lipid maldigestion/malabsorption conditions, LC-PUFA MAG oil increased LC-PUFA levels in erythrocytes, plasma, and chylomicrons to a greater extent than TAG. The study concludes that MAG oil (MAG-O3) might require minimal enzymatic digestion prior to intestinal uptake and transfer across the epithelial barrier. (18)
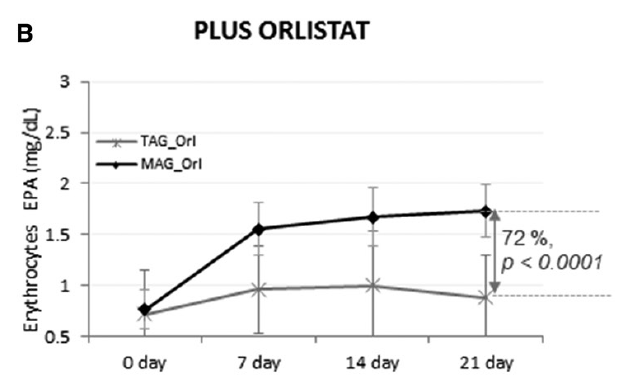
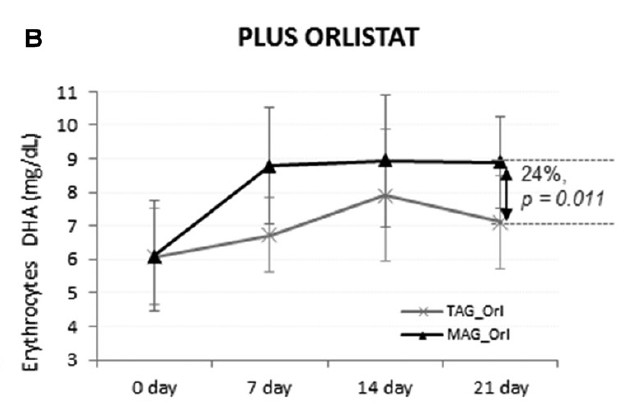
Figure 4: Accretion of LC-PUFAs at days 0, 7, 14, and 21. EPA and DHA content after MAG-enriched oil and TAG supplementation in erythrocytes in the obese with Orlistat group. Results are expressed in mg/mL.
Figure 5: Acute effect: pharmacokinetic results, EPA (A) and DHA
(B) in chylomicrons, AUC over 10 h postprandial. Results are expressed
as milligrams per deciliter × hours.
Clinical Bioavailability Study 5
The fifth study explored the bioavailability of MAG-O3 in humans with different metabolic capacity, and how it compared with other forms of Omega-3. First, the authors aimed to demonstrate that sn-1(3)-monoacylglycerol oil (MAG-O3) might be more bioavailable when compared to ethyl ester and FFA carriers in healthy subjects under low fat diet (clinical trial A). Second, the bioavailability of the MAG was compared to TAG oil in overweight/obese healthy subjects under a low-fat diet by measuring DHA/EPA levels in blood plasma (clinical trial B). Third, the bioavailabilities of the MAG and TAG oils were compared in cystic fibrosis patients with known exocrine pancreatic insufficiency. The absorption of LC-PUFA was compared by the measurement of DHA and EPA in plasma and erythrocytes (clinical trial C).
Authors show in humans that a “pre-digested” Omega-3-sn-1(3)-monoacylglycerol lipid structure (MAG-O3) has a significantly greater absorption at high therapeutic doses (2.9 g/day) than the most commonly Omega-3-ethyl ester (3.1 g/day) form (used for the treatment of hypertriglyceridemia), and a comparable profile to other pre-digested Omega-3 free fatty acids (OM3-FFA) structure (3.2 g/day). Nutritional supplement doses of MAG resulted in similar increases in Omega-3 blood level, compared to Omega-3 triacylglycerols (OM3-TAG) supplements in obese subjects (1.2 g/day) under low fat diet, and in children with cystic fibrosis (1.0 g/day). These results suggest that both forms of pre-digested MAG-O3 and OM3-FFA are effectively absorbed and re-incorporated effectively into triacylglycerols inside the enterocytes, before being exported into the chylomicrons lipid transport system. The study concluded that the pre-digested MAG-O3 might provide a more effective therapy in severe cardiovascular conditions where high doses of Omega-3 are required and a low-fat diet is indicated, which limited digestive lipase activity. (19)
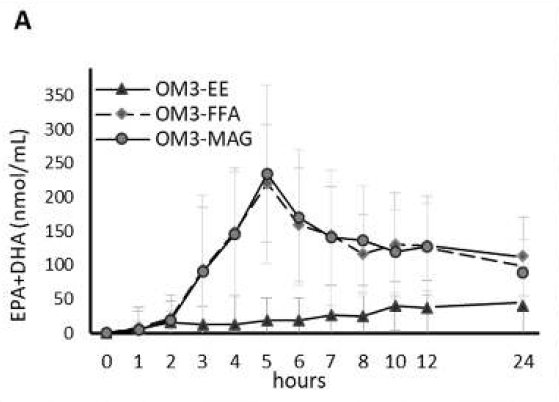
Figure 6: Clinical trial A: Healthy Population. Acute effect: Pharmacokinetic results (baseline-adjusted), EPA + DHA in Plasma, AUC over 24 h postprandial.
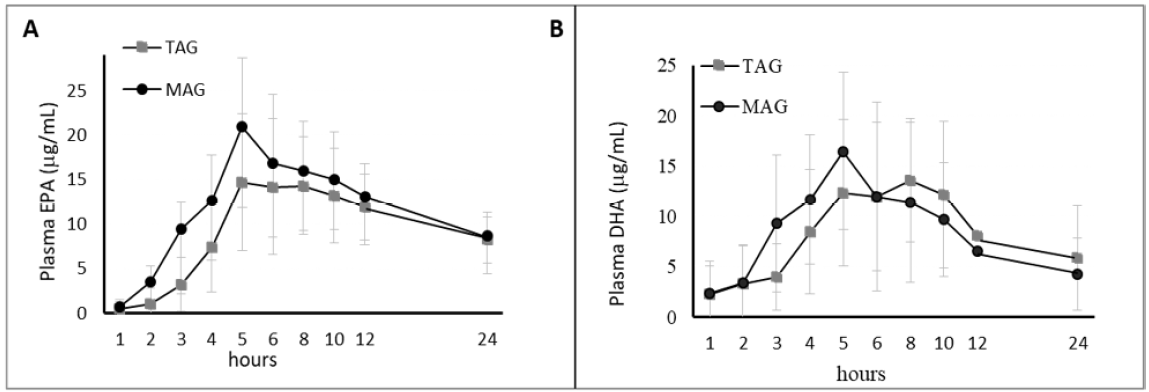
Figure 7: Clinical trial B: Obese Population. Acute effect: Pharmacokinetic results (baseline-adjusted), EPA (A) + DHA (B) in Plasma, AUC over 24 h postprandial (upper panel). Results are expressed in ug/mL.
Conclusion
In conclusion, these studies provide strong evidence that the MAG form of Omega-3 (MAG-O3) is a more effective carrier of Omega-3 compared to the TG and EE forms, particularly for populations that suffer from lipid malabsorption or obesity. The MAG form of Omega-3 has been shown to have higher absorption rates and to be more effective in increasing the Omega-3 Index, a risk factor for coronary heart disease. Additionally, the MAG form of Omega-3 has similar or fewer side effects compared to the EE and TG forms. Given the health benefits of Omega-3, healthcare providers should consider recommending Monoglyceride Omega-3 (MAG-O3) supplements as a form of Omega-3 supplementation to their patients.
References
1. Musa-Veloso K, Binns MA, Kocenas AC, Poon T, Elliot JA, Rice H, Oppedal-Olsen H, Lloyd H, Lemke S. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid dose-dependently reduce fasting serum triglycerides. Nutr Rev. 2010 Mar;68(3):155-67. doi: 10.1111/j.1753-4887.2010.00272.x. PMID: 20384846. (link)
2. Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. (link)
3. Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. (link)
4. Davidson, M.H.; Stein, E.A.; Bays, H.E.; Maki, K.C.; Doyle, R.T.; Shalwitz, R.A.; Ballantyne, C.M.; Ginsberg, H.N. COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Effcacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2007, 29, 1354–1367. (link)
5. Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. (link)
6. Oman, E.; Marenco, T.; Ferber, S.; Johnson, J.; Kling, D.; Curcio, D.; Davidson, M. Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: The ECLIPSE II study. Vasc. Health Risk Manag. 2013, 9, 563–573. (link)
7. Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A Randomized clinical trial to determine the effcacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014, 13, 1–13. (link)
8. Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. (link)
9. Maki, K.C.; Palacios, O.M.; Bell, M.; Toth, P.P. Use of supplemental long-chain omega-3 fatty acids and risk for cardiac death: An updated meta-analysis and review of research gaps. J. Clin. Lipidol. 2017, 11, 1152–1160.e2. (link)
10. Mu, H., and C. E. Høy. 2004. The digestion of dietary triacylglycerols. Prog. Lipid Res. 43: 105–133. (link)
11. Christensen, M. S., C. E. Høy, C. C. Becker, and T. G. Redgrave. 1995. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 61: 56–61. (link)
12. Innis, S. M., and R. Dyer. 1997. Dietary triacylglycerols with palmitic acid (16:0) in the 2-position increase 16:0 in the 2-position of plasma and chylomicron triacylglycerols, but reduce phospholipid arachidonic and docosahexaenoic acids, and alter cholesteryl ester metabolism in formula-Fed piglets. J. Nutr. 127: 1311–1319. (link)
13. Valenzuela, A., V. Valenzuela, J. Sanhueza, and S. Nieto. 2005. Effect of supplementation with docosahexaenoic acid ethyl ester and sn-2 docosahexaenyl monoacylglyceride on plasma and erythrocyte fatty acids in rats. Ann. Nutr. Metab. 49: 49–53. (link)
14. Langlois K, Ratnayake WM. Omega-3 Index of Canadian adults. Health Rep. 2015 Nov;26(11):3-11. PMID: 26583692. (link)
15. Chevalier L, Plourde M. Comparison of pharmacokinetics of omega-3 fatty acid supplements in monoacylglycerol or ethyl ester in humans: a randomized controlled trial. Eur J Clin Nutr. 2021 Apr;75(4):680-688. doi: 10.1038/s41430-020-00767-4. Epub 2020 Oct 3. PMID: 33011737; PMCID: PMC8035073. (link)
16. Chevalier L, Vachon A, Plourde M. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J Nutr. 2021 May 11;151(5):1111-1118. doi: 10.1093/jn/nxaa458. PMID: 33564872; PMCID: PMC8112767. (link)
17. Unpublished Comparative human pilot study evaluating three different fish oil forms and their impact on the Omega-3 index.
https://clinicaltrials.gov/ct2/show/NCT04159532?term=NCT04159532&draw=2&rank=1
18. Cruz-Hernandez C, Destaillats F, Thakkar SK, Goulet L, Wynn E, Grathwohl D, Roessle C, de Giorgi S, Tappy L, Giuffrida F, Giusti V. Monoacylglycerol-enriched oil increases EPA/DHA delivery to circulatory system in humans with induced lipid malabsorption conditions. J Lipid Res. 2016 Dec;57(12):2208-2216. doi: 10.1194/jlr.P070144. Epub 2016 Oct 5. PMID: 27707818; PMCID: PMC5321218. (link)
19. Cuenoud B, Rochat I, Gosoniu ML, Dupuis L, Berk E, Jaudszus A, Mainz JG, Hafen G, Beaumont M, Cruz-Hernandez C. Monoacylglycerol Form of Omega-3s Improves Its Bioavailability in Humans Compared to Other Forms. Nutrients. 2020 Apr 7;12(4):1014. doi: 10.3390/nu12041014. PMID: 32272659; PMCID: PMC7230359. (link)

MAG-O3 Monoglyceride Omega-3: Ideal Omega-3 Fish Oil for populations on a low fat diet or that suffers from lipid malabsorption or obesity.